Resolve Quality Issues in Hours, not Days
Uncountable's AI powered LIMS connects test results directly to formulations, raw material lots, and production history, so your team stops chasing context and starts making decisions.
Still managing QC with spreadsheets, disconnected systems, and institutional knowledge? Most teams lose days on root cause analysis, fight inconsistent processes across sites, can't trace quality failures back to formulation changes, and scramble every time an audit lands. Uncountable connects QC directly to product, process, and lifecycle data — so quality issues don't live in isolation.
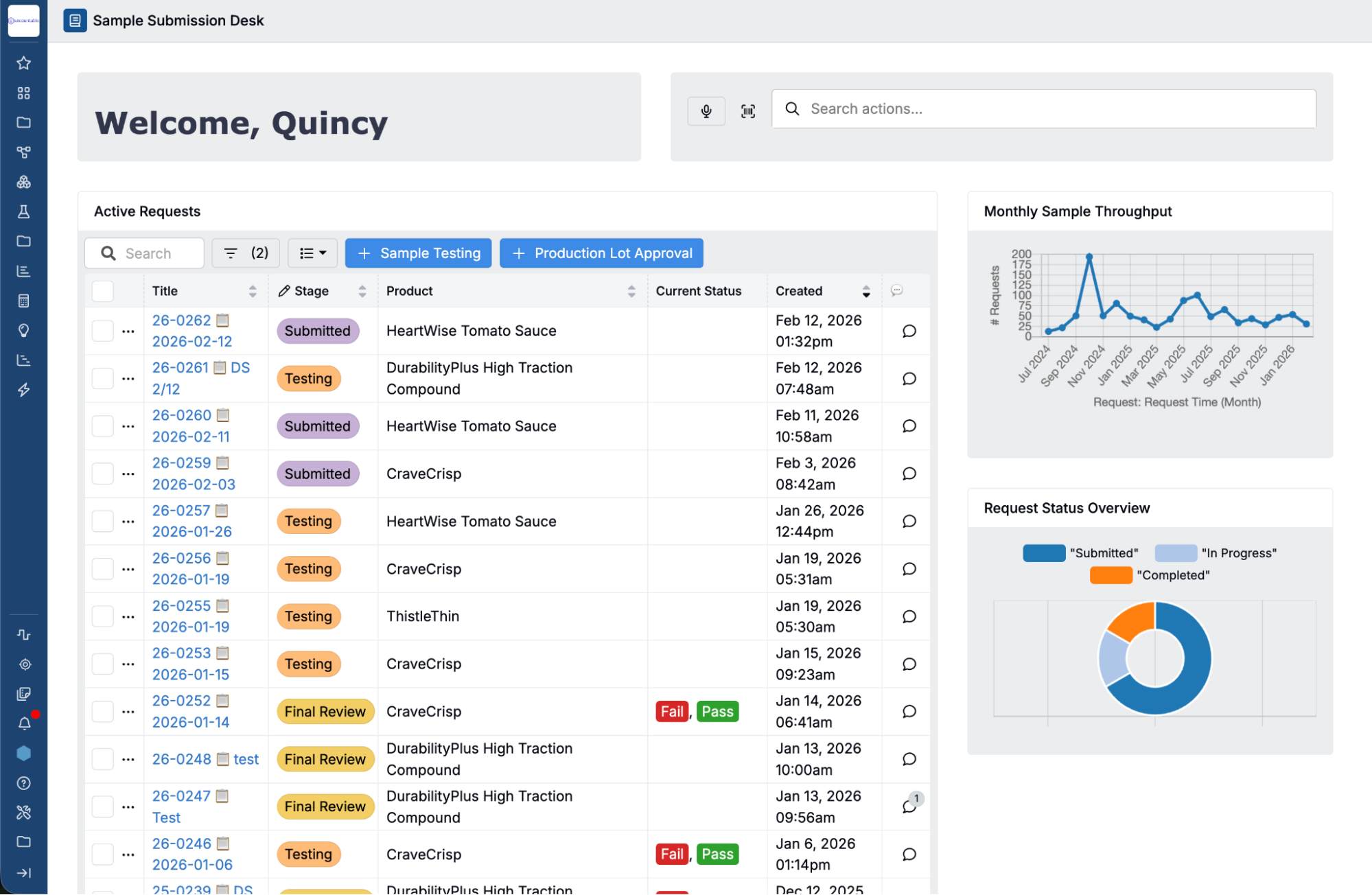
Centralize Quality Data & Documentation
Bring all quality-related data - from test results to deviations and approvals - into a centralized system that supports both R&D and manufacturing teams.
Unified Hub for Quality Management
Manage quality events, control plans, test results, and documentation in a single platform.
Structured, Searchable Records
Quickly retrieve past results, reports, and approvals with advanced filtering, ensuring full traceability
Resolve Deviations Faster
Combine visualizations with AI tooling for quicker root cause analysis to quickly fix out of spec and other incidents.
Link Quality to Product Data
Connect test results directly to specific formulations, batches, or production runs for context-rich insights.






A Batch Fails Spec. What Happens Next?
It's 6 AM and Plant B flags an out-of-spec viscosity result on a production batch. Here's what happens in Uncountable versus what your team is probably doing today.
Without Uncountable
Your QC lead opens a spreadsheet, emails the lab for the raw material COAs, waits for R&D to confirm whether the formulation changed recently, and starts manually pulling historical test data to look for a pattern. By mid-afternoon, three people have spent half their day on it. The line is still on hold.
With Uncountable
Your QC lead pulls up the batch, sees the full formulation and raw material lot history linked directly to the test result, and filters for every batch that used the same supplier lot in the last 90 days. The AI flags a correlation between the lot change and viscosity drift across two other products. Root cause identified, CAPA initiated, line released, all before lunch.
Automate Workflows and Reduce Manual Effort
Digitize and automate key quality workflows to reduce human error, ensure consistency, and increase operational efficiency.
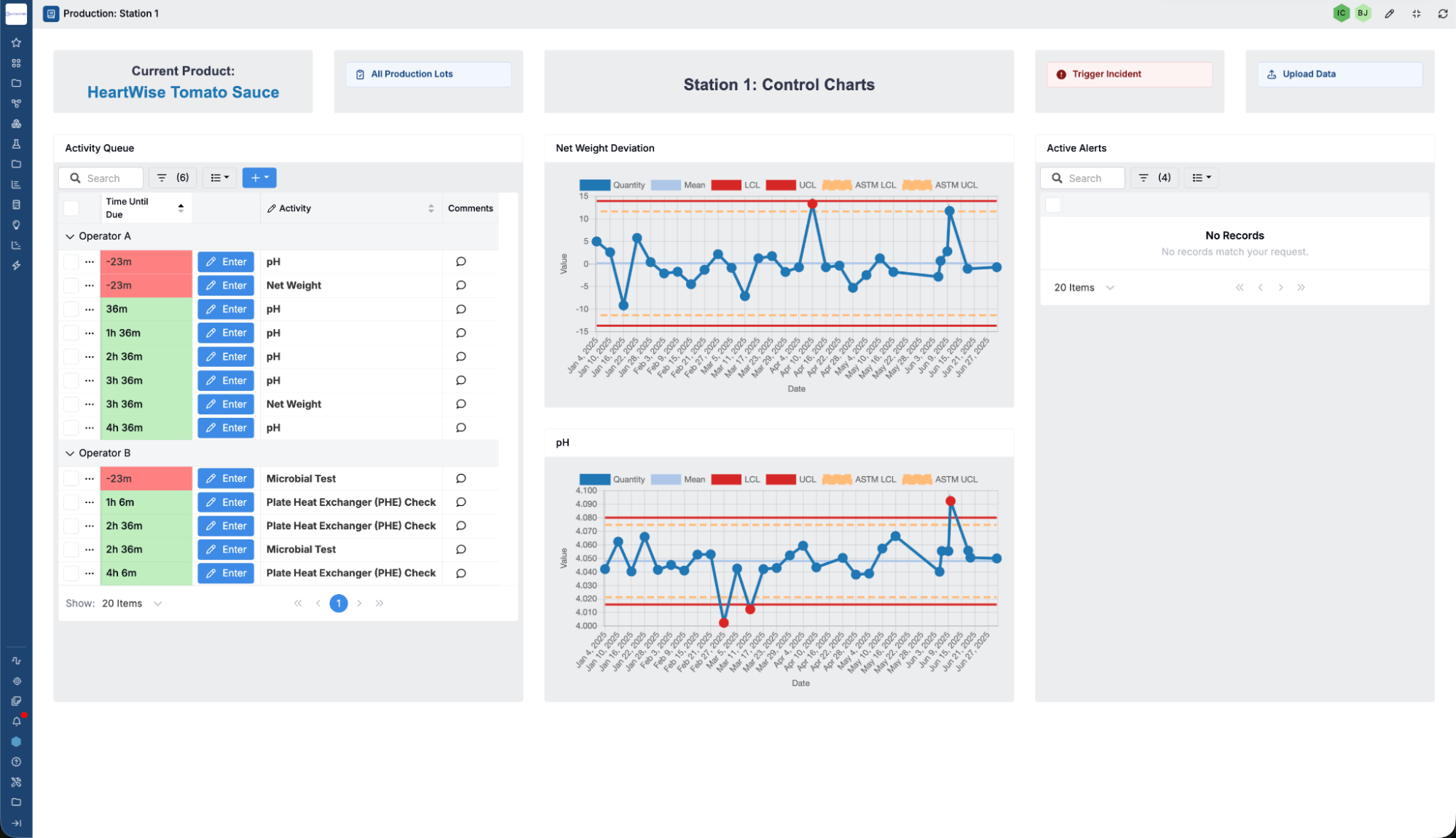
- Workflow Automation — Configure workflows for test reviews, CAPA, and non-conformance management to standardize execution and speed up cycle times.
- Task & Notification Management — Automatically assign tasks and send alerts to keep work moving, prevent bottlenecks, and reduce missed handoffs.
- Reduce Administrative Overhead — Replace spreadsheets and manual tracking with end-to-end digital execution to save time and improve traceability.
Ensure Complete Traceability & Audit-Readiness
Maintain detailed audit trails and version histories for all quality events, results, and approvals — supporting regulatory and internal compliance.
- Built-In Audit Trails — Automatically log every change, action, and decision across the QC LIMS.
- Version Control Across Records — Track iterations of methods, tests, and reports with full visibility.
- Compliance-Ready Documentation — Easily generate exportable reports to meet ISO, GxP, and industry-specific requirements.
- Cross-Site Alignment — Harmonize quality practices across multiple labs or plants to maintain high standards globally.
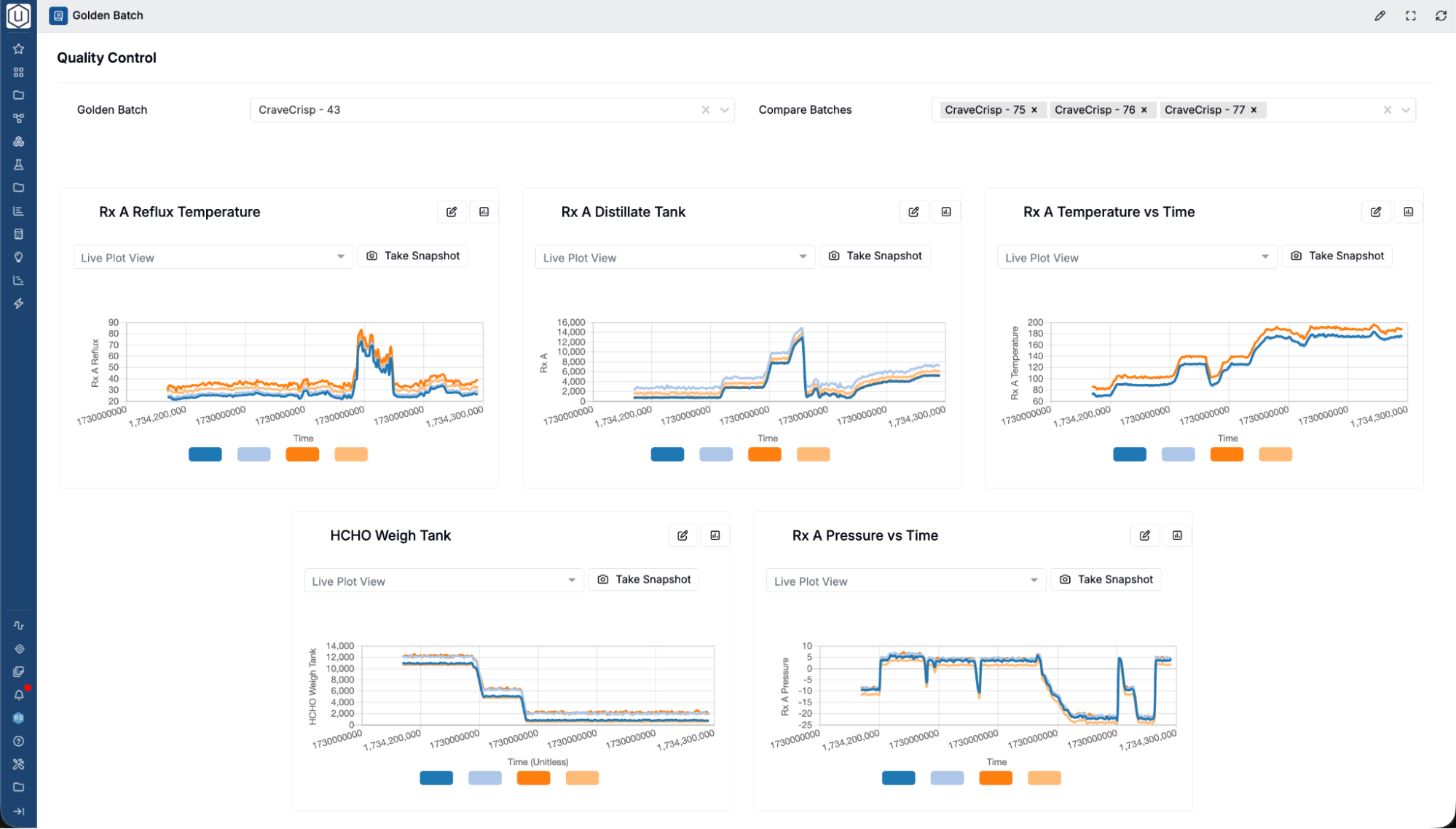
Integrate Seamlessly with R&D & Production Systems
Break down silos between R&D, QC, and manufacturing by integrating QC LIMS with your broader data ecosystem.
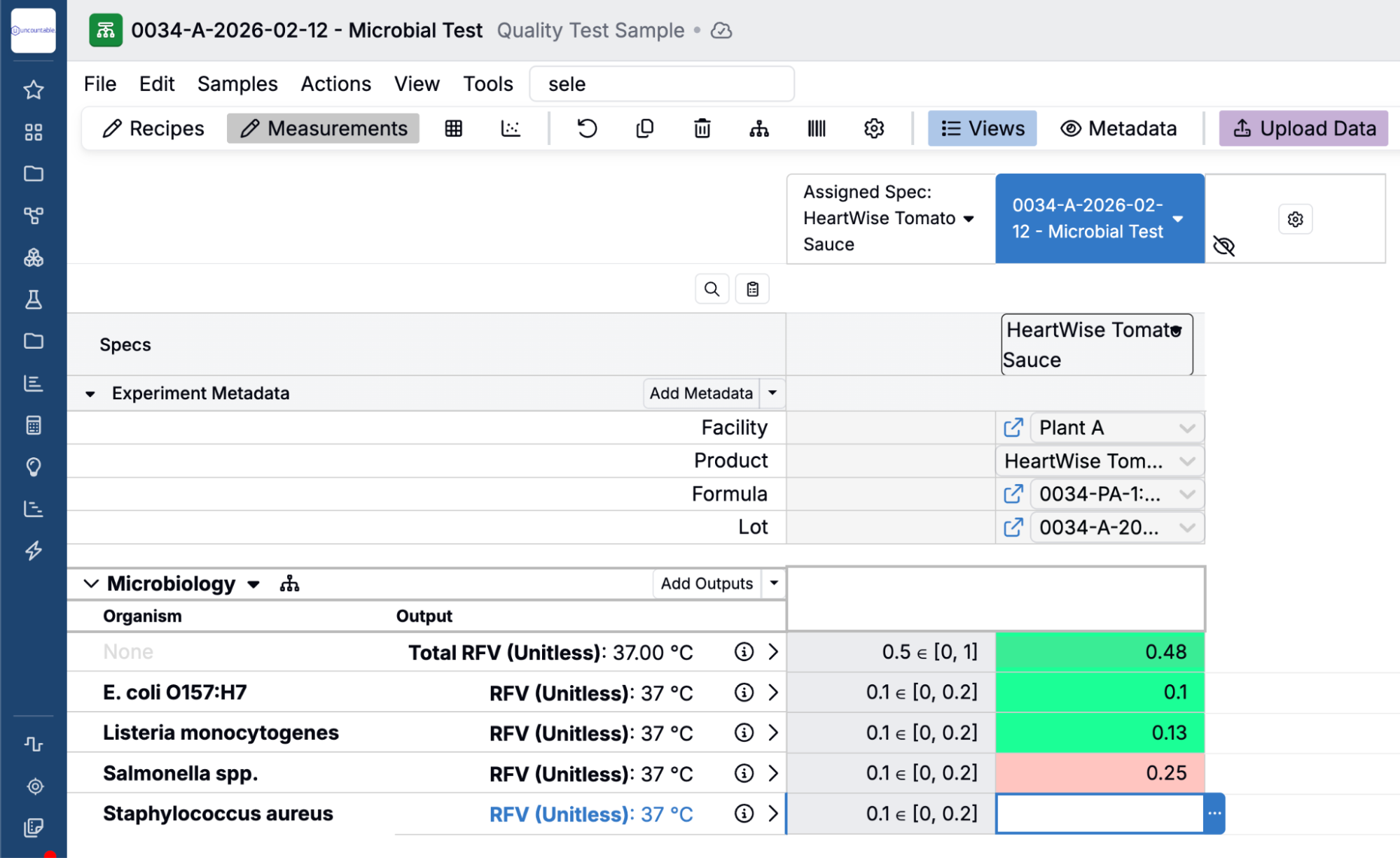
- Built-in QMS & PLM — Link quality events directly to formulations, samples, and experiments. Trace non-conformities through a full CAPA process.
- Instrument & ERP Integration — Automatically pull in results and sync with operational systems, including all leading ERPs.
- Unified Data Backbone — Reduce duplication and ensure consistency across platforms and teams.
Uncountable’s QC LIMS Solution vs. Other QC LIMS
Uncountable's integrated QC LIMS offers the same flexibility as standalone QC LIMS systems, but simplifies & eliminates the error-prone and manual work required to attain accurate & comprehensive insights you need.
Uncountable's Integrated QMS
Other QMS Vendors
You Won’t Be Doing This Alone
Switching QC systems in a regulated environment isn’t something you do on a whim. We know that. Uncountable’s implementation team has deployed QC LIMS across single-site labs and multi-plant global operations—and we’ve developed a change management approach tailored to what actually works in manufacturing environments, not what looks good on a project timeline.
A Phased Rollout Built Around Your Reality
Every deployment follows a structured rollout that adapts to your team’s capacity, regulatory requirements, and existing workflows. You won’t be asked to flip a switch—you’ll move through stages designed to build confidence and minimize disruption.
We map your current QC workflows, instruments, data sources, and compliance requirements. We identify what migrates, what changes, and what stays.
QC leads and IT stakeholders participate in workflow mapping sessions. No heavy lifting—just honest conversation about how things actually work today.
We configure your LIMS environment: test methods, spec limits, approval workflows, instrument connections, and role-based access. Structured historical data is migrated and validated.
Your team reviews configurations and validates migrated data against existing records. We provide side-by-side comparison reports.
We run structured UAT scenarios based on real workflows to confirm configurations, calculations, permissions, and integrations work as expected. Issues are logged, prioritized, and resolved before validation.
QC operators and IT stakeholders execute test cases, confirm expected outcomes, and provide feedback on usability and workflow alignment. Your team signs off once the system meets operational requirements.
Your team runs the new system alongside existing processes. We validate that results, workflows, and audit trails match expectations.
QC operators use both systems in parallel for a limited period. Your validation team confirms data integrity and regulatory compliance before cutover.
Full cutover with dedicated support. We embed with your team during the first weeks of live operation to resolve issues in real time.
QC operators use both systems in parallel for a limited period. Your validation team confirms data integrity and regulatory compliance before cutover.
After stabilization, we help you extend the system: adding sites, refining workflows, building custom reports, and connecting additional instruments.
Your team identifies improvement opportunities. We help prioritize and execute.
Ongoing Support That Doesn’t Disappear After Go-Live
Implementation is not a project with a hard end date. After go-live, every customer continues to receive:
A dedicated Account Manager who knows your workflows and your team
Quarterly business reviews to identify optimization opportunities and track ROI
Priority support with SLAs tailored to manufacturing environments
Continuous platform updates with guided rollouts—no surprise breaking changes
What Your Team Is Actually Worried About
We’ve heard every concern. Here’s how we address the ones that come up in almost every conversation.
“Our operators aren’t going to learn a new system overnight.”
They don’t need to. We deliver role-based training—your QC technicians learn the workflows they use daily, not the full admin console. Training is hands-on, in your environment, with your actual data. We also provide on-demand video walkthroughs and a dedicated support channel during the transition.
“We need to stay compliant during the transition.”
The parallel run is specifically designed for this. Your validation team can confirm that audit trails, electronic signatures, and approval workflows meet your regulatory requirements before you decommission anything. We’ve supported transitions under ISO 9001, GxP, and FDA 21 CFR Part 11 environments.
“We can’t afford downtime on the production line.”
You won’t have any. The parallel run phase means your existing system stays operational until your team is fully confident in the new one. Cutover happens on your timeline, not ours.


