Stop Losing Months to Disconnected Product Data
Uncountable's AI-Powered PLM connects product development, scale-up, and manufacturing in one system of record: from formulation notebooks to BOMs to the plant floor

Your Product Data Is Scattered. Your Teams and Budget Pay the Price.
Most manufacturers don’t lose months to a single catastrophic failure. Instead, months are lost due to smaller, incremental failures over time. For example, a reformulation that starts from scratch because no one can find the original trial data. A tech transfer that stalls because the process parameters live in one engineer’s notebook. A regulatory audit that takes three weeks instead of three hours because batch records span four systems and a shared drive.
The cost isn’t just inefficiency. It’s compounding risk.
Every disconnected handoff introduces the chance of error: a wrong revision, a missed spec update, a substitution that wasn’t fully validated. In regulated industries, those errors don’t just delay launches. They trigger recalls, warning letters, and lost customer trust. And the longer your product data stays fragmented, the harder it gets to fix. Teams build workarounds. Workarounds become “standard practice” and are accepted as part of culture. By the time most companies evaluate a PLM, they’re not just replacing a tool—they’re unwinding years of institutional drift.
R&D works in isolation.
Formulation history, experiment results, and design rationale sit in spreadsheets, ELNs, or local files that no one outside the lab can access. When a scientist or engineer leaves, years of institutional knowledge leave with them.
Scale-up reinvents the wheel.
Pilot teams re-derive process parameters that were already optimized at bench scale because the data didn’t transfer cleanly, or at all. Discrete teams rebuild BOMs and specs from incomplete documentation. The result: months of avoidable rework and trial runs that repeat failures the lab already resolved.
Manufacturing gets a partial picture.
Production receives specs with no rationale for why a tolerance was set, no history of what was tried and rejected, no link back to the development record. When something goes wrong on the line, root cause analysis means chasing people, not querying a system.
Quality and regulatory scramble every time.
Auditors ask for change history. Your team spends days stitching together email threads, PDF approvals, and version-ambiguous spreadsheets. Every audit is a fire drill because traceability was never built into the workflow, it was bolted on after the fact.
One System of Record from Lab to Plant
Eliminate the spreadsheets, shared drives, and siloed tools that slow every handoff. Centralize formulations, bills of materials, test data, process parameters, and documentation in a single searchable platform.
Formulation-First Data Model
Manage quality events, control plans, test results, and documentation in a single platform.
Full-Lifecycle Visibility
Trace every product from concept through reformulation, tech transfer, scale-up, and continuous improvement, with complete context on what changed and why.
Cross-Functional Alignment
R&D, quality, manufacturing, and regulatory teams work from the same governed source of truth, so handoffs don’t mean starting over.






Turn Product Data into a Competitive Advantage
All PLMs store data. Uncountable’s AI-native architecture makes it work for you, surfacing patterns across thousands of experiments and production runs that no team could find manually.
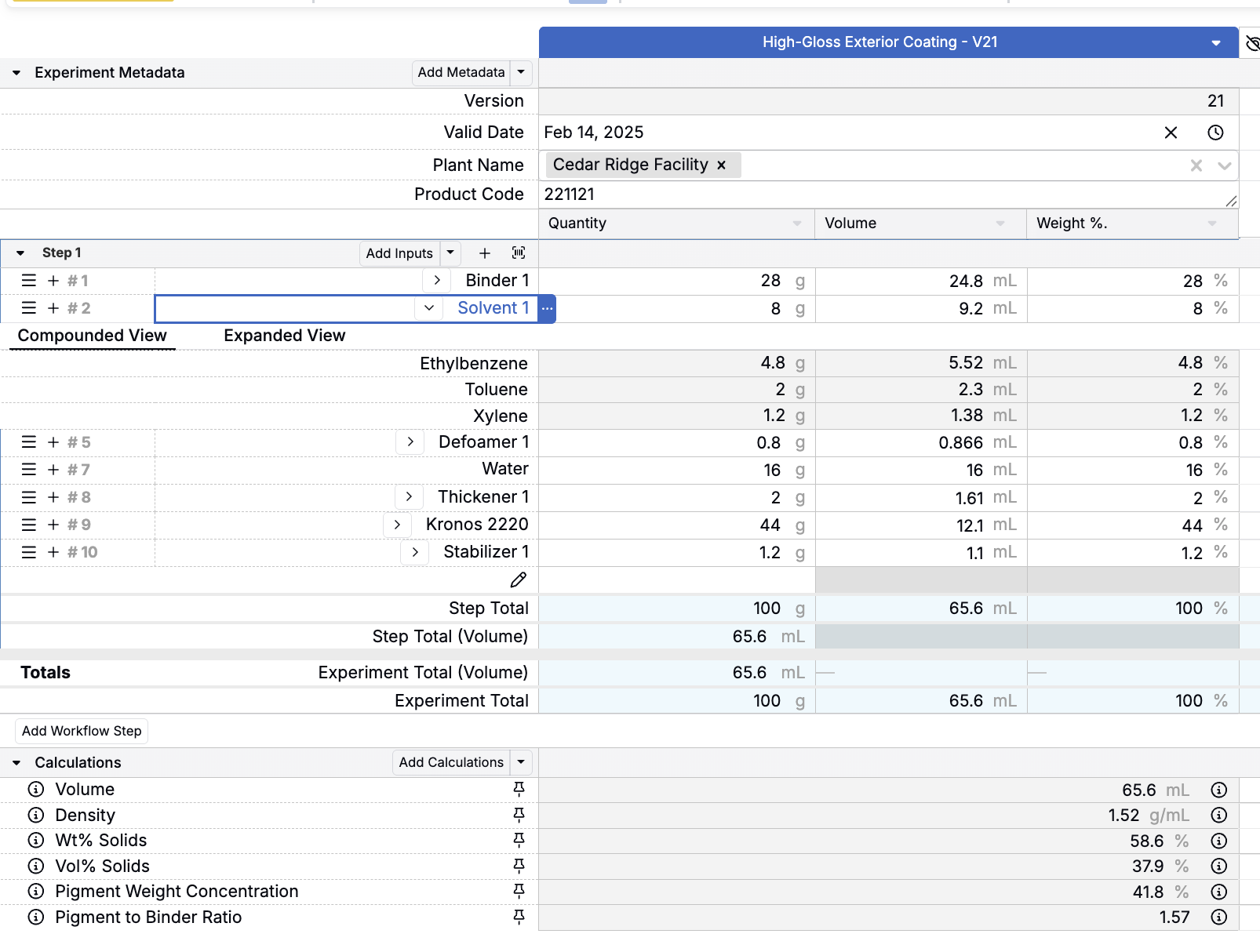
Predictive Formulation Modeling
Use historical data to predict outcomes before running trials. Identify optimal ingredient combinations, material selections, and process parameters to reduce costly experimentation.
Production Pattern Recognition
Detect trends in batch variability, seasonal raw material shifts, and process drift across production lines and sites.
AI-Guided Optimization
Receive recommendations for formulation adjustments, design refinements, process tweaks, and next-best experiments based on your organization's full data history.
Accelerate Product Iteration & Innovation
Reuse proven formulations, designs, and experiment history to reduce rework and bring better products to market faster.
Protect Institutional Knowledge
Capture the reasoning behind formulation and design decisions, so critical knowledge survives team changes and retirements.
Reuse & Iterate Faster
Search across the full experiment and revision history to find proven starting points. Reduce redundant trials and accelerate time-to-market for new products, reformulations, and design iterations.
Manage Change Complexity
Track raw material substitutions, component changes, regulatory-driven updates, and customer-requested modifications with full traceability back to the original product, whether that's a formulation revision or an engineering change order.
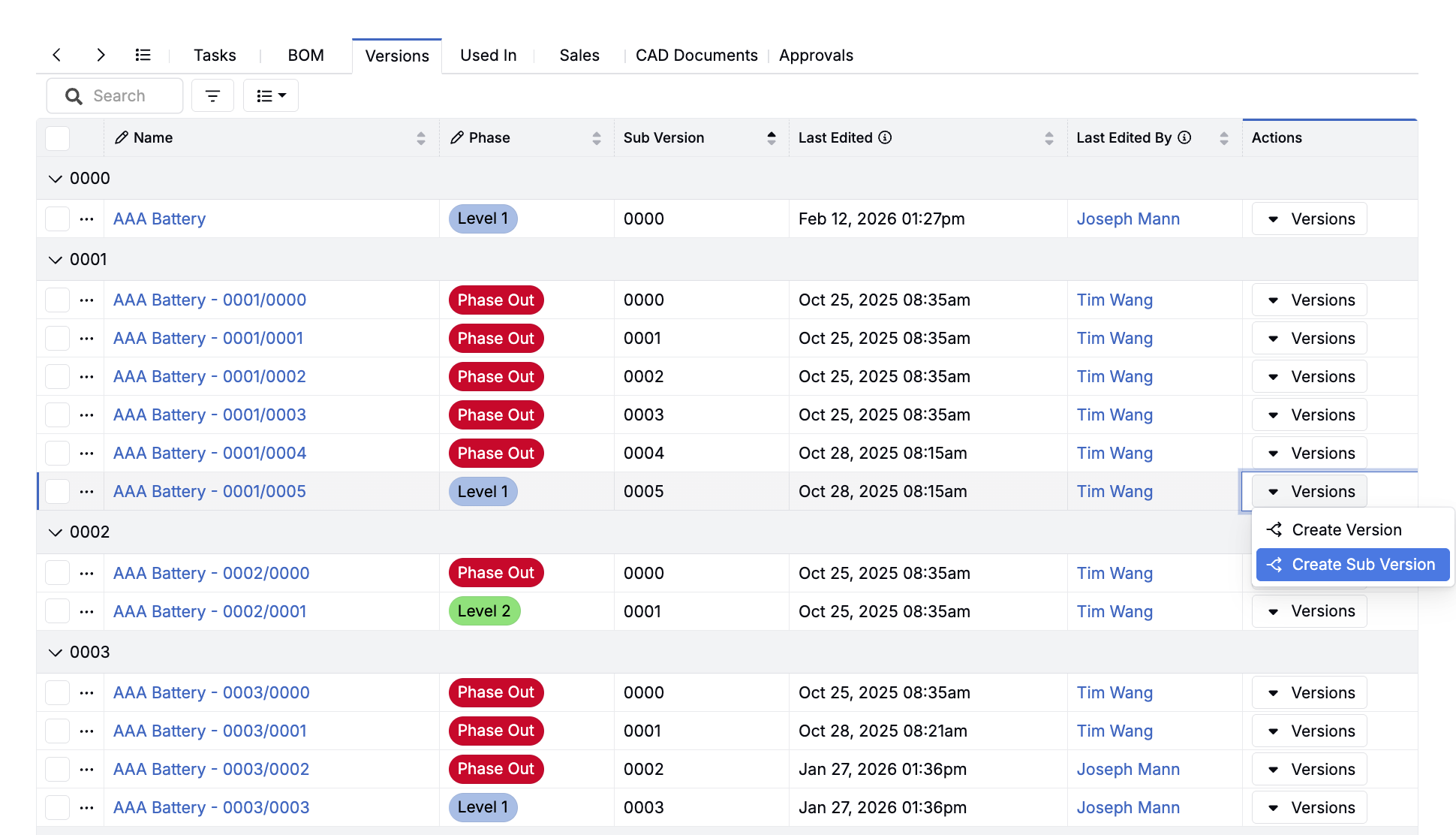
Audit-Ready Traceability, Built In
Regulatory audits and customer inquiries shouldn’t trigger weeks of scrambling. Every change, approval, and decision is tracked automatically.
Version Control & Change History
Automatically track every change with timestamps, ownership, rationale, and supporting data—across every product version.
Regulatory Documentation
Generate audit-ready reports, submission packages, and compliance documentation directly from your product records.
Root Cause Analysis
When batch-to-batch variability occurs, trace backward from production results to process parameters, raw materials, and formulation changes.
Traceability extends into quality control.
Uncountable’s PLM integrates directly with our QC solution, so in-process and final quality data flows back into the product record. Scale faster, reduce risk, and stop losing money to preventable quality and compliance failures.
Bridge the Gap from Bench to Production
Tech transfer failures cost months and millions. Uncountable keeps process parameters, design specs, scale-up data, and manufacturing requirements linked to the original development record across every site.
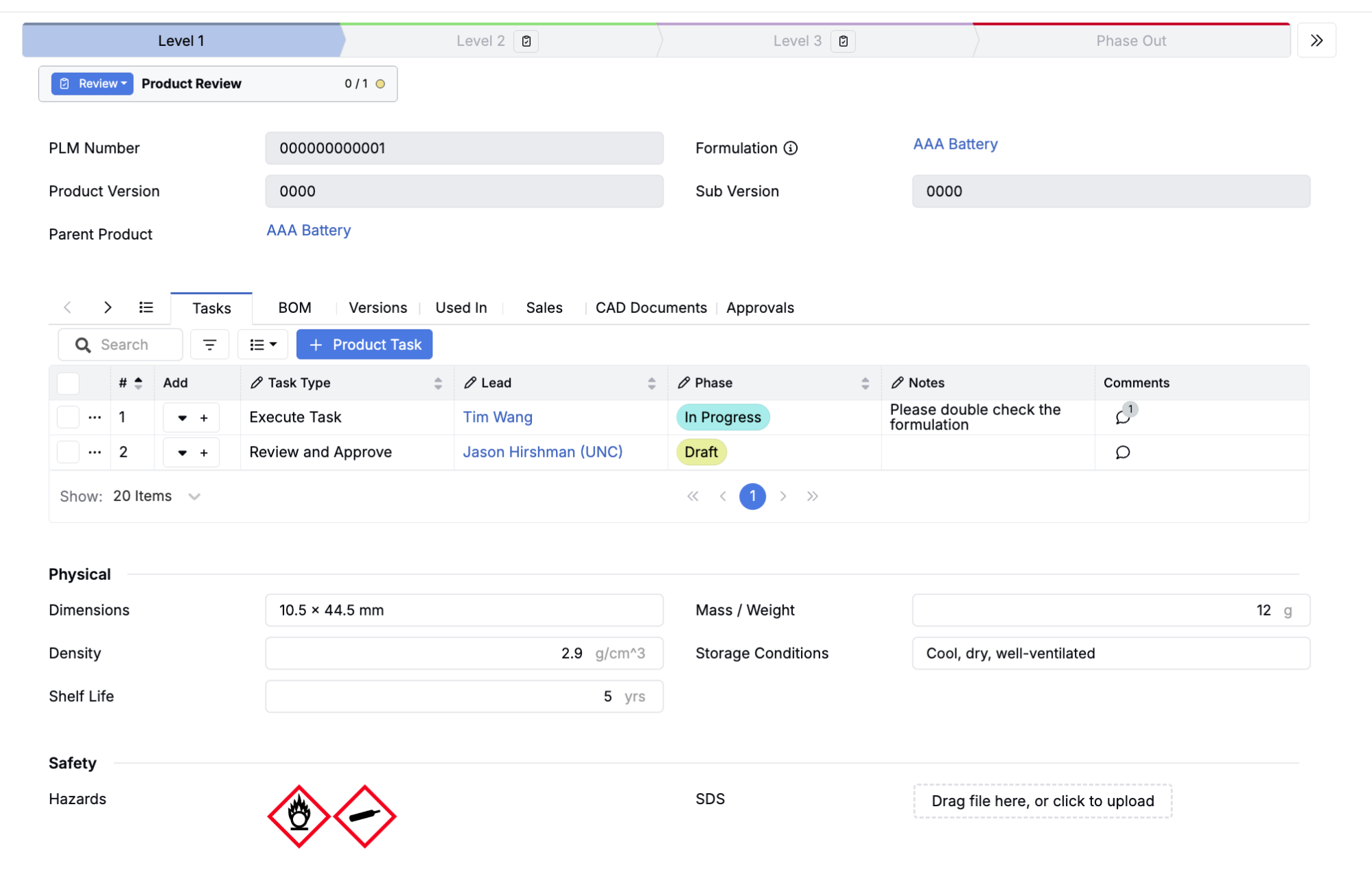
Lab-to-Plant Traceability
Link early-stage development to pilot runs to full production. Trace a bench formulation through scale-up, or follow a prototype design through validation and release. Understand exactly what changed at each stage and why.
Multi-Site Product Governance
Manage product recipes, specifications, and configurations across plants with different equipment, capacities, and supply chains.
Manufacturing Handoff
Give production teams the specs, process parameters, work instructions, and context they need


